Enhance Trial Efficiency
Streamline processes, support adaptive designs, and improve data accuracy with ClinicalHawk's IWRS system.
ClinicalHawk IWRS System:
Elevating Clinical Trial Supply Management
In the dynamic and ever-evolving landscape of clinical trials, efficient supply management is critical to the success of research projects. ClinicalHawk's Interactive Web Response System (IWRS) stands at the forefront of innovation, designed to address the multifaceted challenges of clinical trial supply management with precision and ease. Our system not only streamlines the process but also enhances accuracy, compliance, and overall trial efficiency.
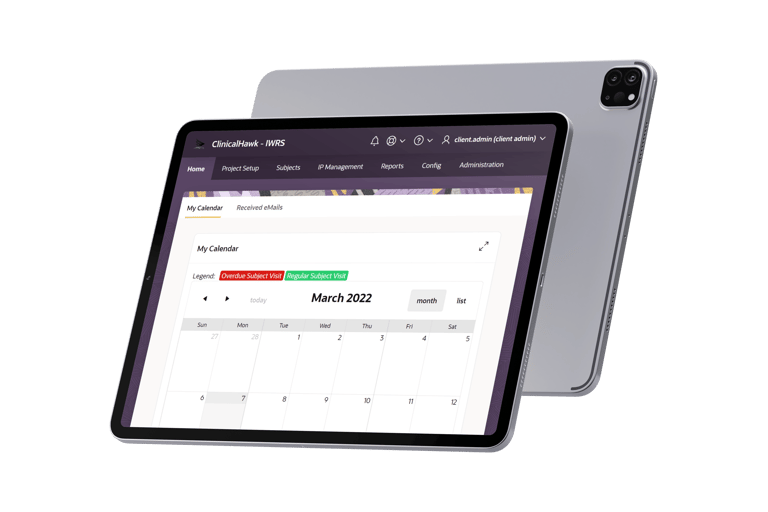
Streamlined Randomization and Supply Management
Randomization and supply management are the cornerstones of a successful clinical trial. ClinicalHawk IWRS provides a robust and flexible platform that supports various randomization methodologies, including simple, block, stratified, and adaptive randomization. Our system is engineered to adapt to the specific needs of your study, ensuring that the right supplies reach the right sites at the right time.
With ClinicalHawk IWRS, you can automate the entire randomization process, reducing the risk of human error and ensuring consistency and reliability. The system's flexibility allows for mid-study adjustments without disrupting ongoing operations, providing an unparalleled level of control over your trial logistics.
Real-Time Inventory Tracking and Management
Effective inventory management is crucial to maintaining the flow of a clinical trial. ClinicalHawk IWRS offers real-time visibility into your inventory, providing detailed tracking of shipments, receipt, and usage of supplies. Our system's advanced analytics and reporting capabilities enable proactive management, allowing you to anticipate and address potential shortages or overstocking issues before they impact your trial.
The real-time inventory tracking feature ensures that all stakeholders have up-to-date information, facilitating better decision-making and coordination. This level of transparency not only enhances operational efficiency but also builds trust among your team and partners.
Enhanced Compliance and Reporting
In the realm of clinical trials, regulatory compliance is paramount. ClinicalHawk IWRS is designed with compliance at its core, incorporating comprehensive reporting and auditing capabilities that facilitate adherence to regulatory requirements. Our system ensures that every action is meticulously documented, providing a clear audit trail for regulatory inspections and internal reviews.
ClinicalHawk IWRS supports compliance with international regulations, including FDA 21 CFR Part 11, EMA, and ICH GCP guidelines. Our built-in validation checks and secure electronic records ensure that your trial data is accurate, reliable, and compliant with industry standards.
User-Friendly Interface and Customization
One of the standout features of ClinicalHawk IWRS is its user-friendly interface. Designed with the end-user in mind, our system simplifies the management of trial supplies, making it accessible to users with varying levels of technical expertise. The intuitive interface allows for easy navigation and quick access to critical information, reducing the learning curve and enhancing user productivity.
Moreover, ClinicalHawk IWRS is highly customizable. Our system can be tailored to meet the specific needs of your clinical trials, whether you are managing a small-scale study or a large, multi-center trial. Customizable dashboards, reports, and alerts ensure that you have the information you need, when you need it, in a format that suits your preferences.
Dedicated Support and Training
At ClinicalHawk, we understand that the successful implementation and operation of our IWRS system depend on the support and training we provide. Our team of experts is dedicated to offering exceptional customer support, ensuring that you have the resources and assistance you need to maximize the benefits of our system.
We offer comprehensive training programs tailored to your team's needs, covering all aspects of the IWRS system from basic operation to advanced features. Our support team is available to assist you with any questions or issues that arise, providing timely and effective solutions to keep your trials running smoothly.
Advanced Interactive Web Response Systems
To provide a more in-depth understanding of the capabilities of ClinicalHawk IWRS, let's explore some of its advanced features



Integrated Temperature Monitoring
For trials involving temperature-sensitive products, ClinicalHawk IWRS includes integrated temperature monitoring. This feature ensures that supplies are stored and transported within the required temperature range, maintaining their integrity and effectiveness.
Automated Supply Resupply
ClinicalHawk IWRS automatically calculates resupply needs based on usage patterns and predefined thresholds. This automation minimizes the risk of supply shortages and ensures that sites always have the necessary resources to continue their work.


Site-Specific Inventory Management
ClinicalHawk IWRS provides detailed inventory management at the site level, allowing you to track and manage supplies for each site individually. This granularity ensures that each site receives the appropriate amount of supplies based on its specific needs.


Adaptive Randomization
Our system supports adaptive randomization, allowing you to adjust randomization procedures based on interim analysis and trial progress. This flexibility enhances the efficiency and effectiveness of your trials, ensuring that they remain responsive to emerging data.


Blinding and Unblinding Management
ClinicalHawk IWRS includes robust blinding and unblinding management capabilities, ensuring that the integrity of your trial is maintained. Our system supports various blinding methods and provides secure and controlled unblinding processes when required.


Multi-Language Support
ClinicalHawk IWRS supports multiple languages, making it suitable for global trials involving diverse participant populations. This feature enhances accessibility and ensures that all users can interact with the system in their preferred language.

Integration with Other Systems
ClinicalHawk IWRS can seamlessly integrate with other clinical trial systems, including electronic data capture (EDC), and clinical trial management systems (CTMS). This integration ensures a unified and cohesive approach to trial management, enhancing data consistency and operational efficiency.
ClinicalHawk IWRS stands as a testament to our commitment to innovation and excellence in clinical trial supply management. Our system offers a comprehensive solution that addresses the complexities and challenges of managing clinical trial supplies with unmatched efficiency, accuracy, and compliance.
By choosing ClinicalHawk IWRS, you are not only investing in a state-of-the-art system but also partnering with a team dedicated to your success. Our robust features, user-friendly interface, and exceptional support ensure that you have the tools and resources you need to conduct.
Frequently asked questions
What is the ClinicalHawk IWRS system?
The ClinicalHawk IWRS (Interactive Web Response System) is a robust tool designed to manage and streamline various aspects of clinical trials, including randomization, drug inventory management, and real-time data reporting.
How does the IWRS system improve clinical trial efficiency?
Our IWRS system provides real-time insights and dynamic control over trial processes, enabling data-driven decision-making, reducing errors, and accelerating trial timelines.
Is the IWRS system customizable for different studies?
Yes, ClinicalHawk's IWRS is highly customizable to meet the unique requirements of different clinical studies, ensuring it aligns perfectly with your trial protocols and workflows.
How secure is the data within the IWRS system?
Security is our top priority. The IWRS system employs advanced encryption and complies with industry standards and regulations to protect sensitive trial data.
Can the IWRS system integrate with other eClinical tools?
Absolutely. Our IWRS system seamlessly integrates with other ClinicalHawk eClinical solutions, including eTMF, EDC, and document automation, ensuring a unified and efficient trial management experience.
What kind of support and training does ClinicalHawk provide?
ClinicalHawk offers comprehensive support and training, including onboarding, user training sessions, and 24/7 technical support to ensure smooth operation and quick resolution of any issues.
Is the IWRS system cloud-based?
Yes, ClinicalHawk's IWRS system is cloud-based, offering flexibility, scalability, and remote access, making it ideal for managing multi-site and global clinical trials.
How does the IWRS system handle randomization?
The system uses advanced algorithms to ensure proper randomization, minimizing bias and ensuring the reliability of trial results.
What are the benefits of using ClinicalHawk's IWRS over other systems?
ClinicalHawk's IWRS stands out due to its intuitive interface, real-time data access, robust security features, and seamless integration with other eClinical tools, providing a comprehensive solution for efficient trial management.
How can I get a demo or trial of the IWRS system?
You can request a demo or by contacting our sales team through by reaching out to us at sales@N2NAcers.com. We will be happy to arrange a personalized demonstration tailored to your study needs.
ClinicalHawk: Empowering Clinical Trials Through Innovation and Integration with tools that enhance patient care, streamline clinical trials, and enable data-driven decision-making.
Clinical Software Solutions
Connect with Us Today
© 2024 ClinicalHawk Systems - Cloud-Based Software for the Worldwide Life Sciences Industry.
All rights reserved. Privacy Policy Terms & Conditions Powered by N2NAcers
Phone – (+91) 910 476 4988
e-Mail Us – Sales@N2NAcers.com
WhatsApp – (+91) 910 476 4988
