ClinicalHawk EDC System:
Your Comprehensive Solution for
Clinical Data Management
In the dynamic world of clinical trials, the need for robust and efficient data management solutions has never been more critical. ClinicalHawk's Electronic Data Capture (EDC) system stands out as a premier choice, tailored to meet the rigorous demands of clinical trial sponsors, Contract Research Organizations (CROs), academic researchers, and pharmaceutical companies. Our EDC system is designed to enhance data accuracy, streamline workflows, and ensure regulatory compliance, all while being user-friendly and cost-effective.
Transform Data Handling
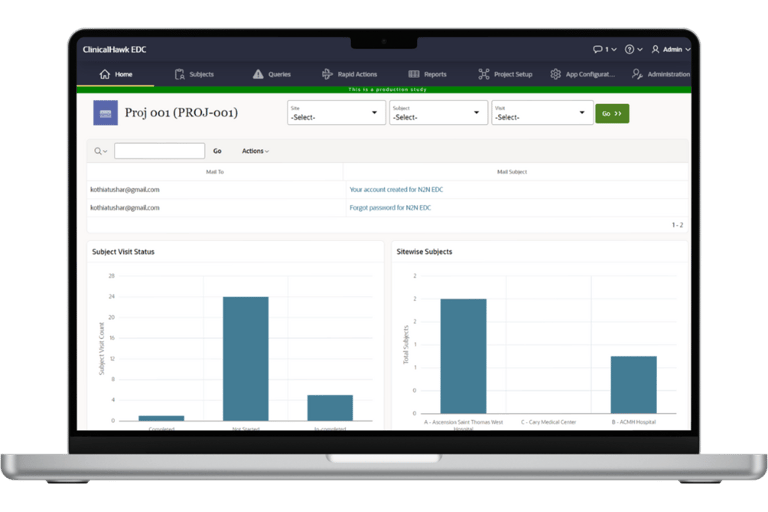
Key Features and Benefits
ClinicalHawk's EDC system boasts an intuitive, user-friendly interface that simplifies data entry and minimizes errors. The system is designed to be accessible to users of all technical levels, ensuring a smooth learning curve and efficient adoption.
Advanced Data Collection and Management
Intuitive Interface
Real-Time Data Access
Our cloud-based platform allows for real-time access to data, facilitating timely decision-making and accelerating the pace of your clinical trials. This feature is particularly beneficial for multi-site studies, where data integration and synchronization are crucial.
Enhanced Data Security and Compliance
Robust Security Measures
ClinicalHawk prioritizes data security, implementing stringent measures to protect sensitive information. Our EDC system is compliant with industry standards such as GDPR, HIPAA, and 21 CFR Part 11, ensuring that your data is secure and your studies are compliant.
Audit Trails and Data Integrity
Comprehensive audit trails and automated data validation checks are integral to our system, maintaining data integrity and providing a clear record of all data modifications.
Customizable and Scalable
Flexible Configurations
The ClinicalHawk EDC system is highly customizable, allowing you to tailor it to the specific needs of your study. Whether you are conducting a small pilot study or a large-scale clinical trial, our system can be configured to meet your requirements.
Scalability
As your study grows, our EDC system scales seamlessly, accommodating increasing data volumes and additional users without compromising performance.
Streamlined Workflow and Collaboration
Efficient Workflow Management
Our EDC system streamlines workflow management, reducing administrative burdens and freeing up valuable time for your research team. Automated workflows and task assignments ensure that your study progresses smoothly and efficiently.
Collaborative Tools
Enhance collaboration among your team with integrated tools that facilitate communication and data sharing. Our platform supports role-based access, ensuring that team members can access the information they need while maintaining data security.
Comprehensive Support and Training
Personalized Support
At ClinicalHawk, we are committed to providing exceptional support to our clients. Our dedicated support team is available to assist you with any technical issues or questions you may have, ensuring that your study runs smoothly from start to finish.
Training Resources
We offer comprehensive training resources, including webinars, tutorials, and user guides, to help you get the most out of our EDC system. Our goal is to empower you to utilize our platform to its full potential.




Optimizing CRO Operations


Accelerating Drug Development
Pharmaceutical companies have leveraged ClinicalHawk's EDC system to streamline their drug development processes, resulting in faster time-to-market for new therapies. Our system's real-time data access and robust security measures have been instrumental in achieving these outcomes.
ClinicalHawk EDC
Use Cases
Enhancing Academic Research
CROs have utilized our EDC system to optimize their operations, improve data quality, and enhance collaboration with sponsors. The system's audit trails and data validation features have been particularly valuable in ensuring compliance and maintaining data integrity.
Academic researchers have benefited from our EDC system's customizable configurations and intuitive interface, enabling them to efficiently manage complex clinical studies. The system's scalability has also allowed them to expand their research projects with ease.
ClinicalHawk's EDC system is a powerful, comprehensive solution designed to meet the diverse needs of clinical trial stakeholders. With its advanced data management capabilities, robust security measures, and user-friendly interface, our EDC system empowers you to conduct efficient, compliant, and successful clinical trials. Choose ClinicalHawk and experience the difference in clinical data management excellence.
For more information and to schedule a demo, click here or contact our sales team today.
Frequently asked questions
What is an Electronic Data Capture (EDC) system?
An EDC system is a software solution designed to collect, manage, and store data generated during clinical trials. It replaces traditional paper-based methods, enhancing data accuracy and efficiency.
How does ClinicalHawk's EDC system ensure data security?
ClinicalHawk's EDC system employs robust security measures, including encryption, secure access controls, and compliance with industry standards such as GDPR, HIPAA, and 21 CFR Part 11.
Can the ClinicalHawk EDC system be customized to suit my study's specific needs?
Yes, our EDC system is highly customizable, allowing you to tailor configurations, forms, and workflows to meet the unique requirements of your study.
Is the ClinicalHawk EDC system scalable for larger studies?
Absolutely. Our EDC system is designed to scale seamlessly, accommodating increasing data volumes and additional users without compromising performance.
What kind of support and training does ClinicalHawk offer?
ClinicalHawk provides comprehensive support and training resources, including webinars, tutorials, user guides, and personalized assistance from our dedicated support team.
How quickly can I set up the ClinicalHawk EDC system for my study?
Our EDC system is designed for rapid deployment, allowing you to set up and start using the platform within a few hours.
Can the ClinicalHawk EDC system integrate with other clinical trial management systems?
Yes, our EDC system can integrate with various other systems, facilitating seamless data flow and improved efficiency in managing your clinical trial processes.
How does ClinicalHawk's EDC system enhance collaboration among study teams?
Our platform includes integrated collaboration tools and role-based access, enabling team members to communicate effectively and share data securely.
What types of studies can benefit from using ClinicalHawk's EDC system?
The ClinicalHawk EDC system is suitable for a wide range of studies, including pharmaceutical trials, academic research, multi-site studies conducted by CROs, observational, and RWE trails.
How does the system handle audit trails and data integrity?
ClinicalHawk's EDC system maintains comprehensive audit trails and automated data validation checks, ensuring data integrity and providing a clear record of all data modifications.
Is there a free trial available for the ClinicalHawk EDC system?
For information on trials and demos, please click here or contact our sales team.
What are the pricing options for the ClinicalHawk EDC system?
Pricing is based on the specific needs and scale of your study. Please contact our sales team for a customized quote.
How does ClinicalHawk ensure the system remains up-to-date with regulatory requirements?
We continuously monitor regulatory changes and update our EDC system to comply with the latest standards and guidelines.
Can data be accessed remotely using the ClinicalHawk EDC system?
Yes, as a cloud-based platform, our EDC system allows for remote data access from any device with an internet connection.
What sets ClinicalHawk's EDC system apart from other EDC solutions?
ClinicalHawk's EDC system combines advanced data management features, robust security, user-friendly interface, and comprehensive support, making it a superior choice for clinical trials.
How does ClinicalHawk's EDC system handle data backups and disaster recovery?
ClinicalHawk's EDC system includes automated data backups and a robust disaster recovery plan to ensure data integrity and availability. Regular backups are performed, and in the event of a system failure, our recovery protocols ensure minimal downtime and data loss.
ClinicalHawk: Empowering Clinical Trials Through Innovation and Integration with tools that enhance patient care, streamline clinical trials, and enable data-driven decision-making.
Clinical Software Solutions
Connect with Us Today
© 2024 ClinicalHawk Systems - Cloud-Based Software for the Worldwide Life Sciences Industry.
All rights reserved. Privacy Policy Terms & Conditions Powered by N2NAcers
Phone – (+91) 910 476 4988
e-Mail Us – Sales@N2NAcers.com
WhatsApp – (+91) 910 476 4988
