ClinicalHawk eTMF System:
Optimize, Simplify, and Elevate Your Clinical Trials
In the rapidly evolving landscape of clinical research, efficient document management is critical. The ClinicalHawk eTMF (electronic Trial Master File) system is designed to streamline this process, offering a robust, cloud-based solution that caters to the needs of clinical trial sponsors, Contract Research Organizations (CROs), academic researchers, and pharmaceutical companies. Our system not only ensures compliance and security but also enhances productivity, making it an indispensable tool for managing clinical trials.
Revolutionize Document Management
Cloud-Based Accessibility
The ClinicalHawk eTMF system is hosted on a secure cloud platform, providing unparalleled accessibility. Users can access the system from anywhere in the world, using any device—be it a laptop, mobile phone, or tablet. This flexibility ensures that your team can collaborate seamlessly, regardless of their location.


Key Features and Benefits
Cost-Effective Solution
In an industry where budget constraints are a constant challenge, our eTMF system offers a cost-effective solution without compromising on quality. By eliminating the need for expensive on-premises infrastructure and reducing the time spent on manual document handling, ClinicalHawk helps you save both time and money.
Easy to Build and Quick Setup
ClinicalHawk’s eTMF system is designed for ease of use. The intuitive interface allows for easy setup of studies, which can be completed within a few hours. This rapid deployment ensures that your clinical trials can start without unnecessary delays, keeping your research timelines on track.
Custom Report Building
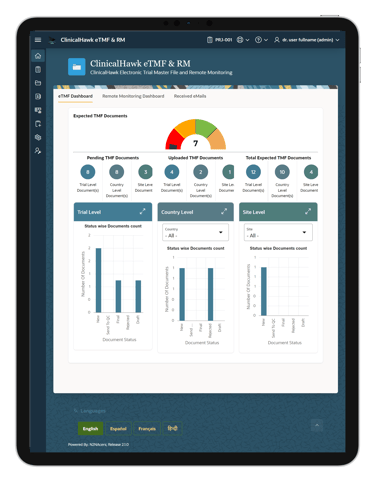
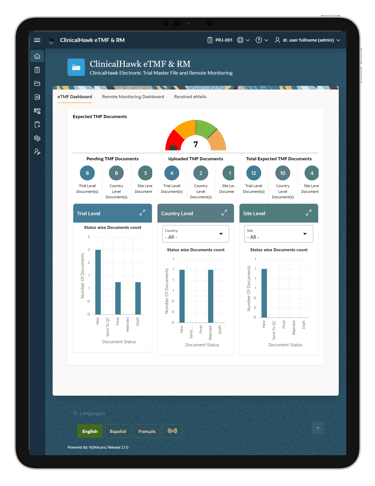
Our eTMF system includes a robust custom report building feature. This allows users to generate tailored reports that meet their specific needs, providing valuable insights and enhancing decision-making processes. Whether you need standard compliance reports or bespoke data analyses, ClinicalHawk has you covered.
Robust Security and Compliance
Security and compliance are paramount in clinical trials. Built on a secure Oracle database, ClinicalHawk’s eTMF system adheres to the highest standards of data protection and regulatory compliance. This ensures that your sensitive trial data is safeguarded against breaches and unauthorized access.

Why Choose ClinicalHawk eTMF System?
ClinicalHawk simplifies document management through automated workflows and intelligent categorization. This ensures that all essential documents are accurately filed and easily retrievable, reducing administrative burden and the risk of misplaced documents.
Streamlined Document Management
Enhanced Collaboration
With its cloud-based platform, ClinicalHawk promotes collaboration across geographically dispersed teams. Real-time document sharing and editing capabilities foster better communication and coordination, leading to more efficient trial management.
Scalability
Our eTMF system is scalable to accommodate the growing needs of your research projects. Whether you are managing a single study or multiple complex trials, ClinicalHawk can scale up to meet your requirements without any compromise on performance.
At ClinicalHawk, we prioritize our clients' needs. Our eTMF system is designed with a client-focused approach, ensuring that every feature and functionality addresses real-world challenges faced by clinical trial professionals. Here’s how we cater to your specific needs:
Continuous Improvement
ClinicalHawk is committed to continuous improvement. We regularly update our eTMF system with new features and enhancements based on user feedback and industry trends. This ensures that you always have access to the latest tools and technologies to support your clinical trials.


Personalized Support
We understand that every clinical trial is unique. That’s why we offer personalized support to help you navigate through the setup and management of your eTMF system. Our expert team is always on hand to provide guidance and resolve any issues promptly.


Client-Focused Approach


Device Compatibility
Our eTMF system is designed to be device-agnostic, meaning it can be accessed from any device with an internet connection. Whether you prefer to work on a laptop, mobile phone, or tablet, ClinicalHawk offers a seamless user experience across all platforms.


Integration Capabilities
To maximize efficiency, ClinicalHawk’s eTMF system integrates seamlessly with other clinical trial management systems (CTMS) and electronic data capture (EDC) tools. This ensures a smooth flow of information between different systems, reducing the risk of data silos and enhancing overall trial management.
Technical Specifications
ClinicalHawk’s eTMF system is built on a robust Oracle database, renowned for its reliability and performance. The cloud-based infrastructure ensures high availability and disaster recovery capabilities, providing peace of mind that your data is always safe and accessible.
Driving Sales Through Client-Focused Benefits
The ClinicalHawk eTMF system is not just a tool; it’s a strategic asset that can drive the success of your clinical trials. Here’s how our client-focused benefits translate into tangible advantages for your organization:


Improved Compliance and Audit Readiness
Our system’s robust security and compliance features ensure that you are always audit-ready. This reduces the risk of regulatory penalties and enhances the credibility of your research.
Cost Savings
By leveraging a cloud-based solution, you can significantly reduce IT costs associated with maintaining on-premises infrastructure. Additionally, the efficiency gains from streamlined document management and automated workflows translate into cost savings throughout the trial lifecycle.
Accelerated Study Start-Up
With our easy-to-build system and quick setup times, you can accelerate the start-up phase of your studies. This means getting your trials up and running faster, reducing time-to-market for your pharmaceutical products.
Enhanced Data Insights
Custom report building capabilities provide you with deeper insights into your trial data. This enables better decision-making and helps identify potential issues before they escalate, ensuring smoother trial operations.
The ClinicalHawk eTMF system is designed to meet the diverse needs of clinical trial professionals. With its cloud-based accessibility, cost-effectiveness, ease of setup, custom reporting, and robust security, it stands out as a premier solution for managing trial master files. By choosing ClinicalHawk, you are investing in a system that enhances efficiency, ensures compliance, and drives the success of your clinical trials.
Experience the future of clinical trial management with ClinicalHawk eTMF. Contact us today to learn more and schedule a personalized demonstration.
Frequently asked questions
What is an eTMF system and why is it important?
An eTMF (electronic Trial Master File) system is a digital solution for managing the essential documents, tasks, and workflows associated with clinical trials. It ensures compliance with regulatory requirements, improves document organization, and enhances collaboration among clinical trial stakeholders.
How does the ClinicalHawk eTMF system ensure data security?
The ClinicalHawk eTMF system is built on a secure Oracle database and hosted on a cloud server with robust security measures, including data encryption, regular security audits, and compliance with industry standards such as GDPR and HIPAA.
Can the ClinicalHawk eTMF system be accessed on mobile devices?
Yes, the ClinicalHawk eTMF system is designed to be accessible from any device, including laptops, mobile phones, and tablets. This ensures that you can manage your clinical trial documents and workflows from anywhere, at any time.
How quickly can the ClinicalHawk eTMF system be set up for a new study?
The ClinicalHawk eTMF system is designed for rapid deployment. Studies can be set up within a few hours, allowing you to start managing your clinical trial documents without unnecessary delays.
What kind of support does ClinicalHawk offer to its eTMF system users?
ClinicalHawk provides personalized support to all eTMF system users. Our expert team is available to assist with system setup, troubleshooting, and any other queries you may have. We are committed to ensuring you get the most out of our eTMF system.
Can I generate custom reports with the ClinicalHawk eTMF system?
Yes, the ClinicalHawk eTMF system includes robust custom report building features. You can create tailored reports to meet your specific needs, providing valuable insights and enhancing decision-making processes.
How does the ClinicalHawk eTMF system help with regulatory compliance?
The ClinicalHawk eTMF system is designed to ensure compliance with regulatory requirements. It includes features such as automated workflows, document version control, audit trails, and secure storage, all of which help maintain regulatory compliance and audit readiness.
Is the ClinicalHawk eTMF system scalable for large clinical trials?
Yes, the ClinicalHawk eTMF system is highly scalable. Whether you are managing a single study or multiple complex trials, our system can scale up to meet your needs without compromising on performance or security.
Can the ClinicalHawk eTMF system integrate with other clinical trial management systems?
Yes, the ClinicalHawk eTMF system is designed to integrate seamlessly with other clinical trial management systems (CTMS) and electronic data capture (EDC) tools. This ensures a smooth flow of information and enhances overall trial management efficiency.
What are the costs associated with the ClinicalHawk eTMF system?
The ClinicalHawk eTMF system offers a cost-effective solution for managing clinical trial documents. For detailed pricing information, please contact our sales team, who will provide you with a tailored quote based on your specific needs and requirements.
ClinicalHawk: Empowering Clinical Trials Through Innovation and Integration with tools that enhance patient care, streamline clinical trials, and enable data-driven decision-making.
Clinical Software Solutions
Connect with Us Today
© 2024 ClinicalHawk Systems - Cloud-Based Software for the Worldwide Life Sciences Industry.
All rights reserved. Privacy Policy Terms & Conditions Powered by N2NAcers
Phone – (+91) 910 476 4988
e-Mail Us – Sales@N2NAcers.com
WhatsApp – (+91) 910 476 4988
